Excited to ha ve Lindsay Cameron from the Neuroscience Graduate Group rotating this quarter!
ve Lindsay Cameron from the Neuroscience Graduate Group rotating this quarter!
Brain Awareness Week!
Jon passed his qualifying exam!
Eden Barragan joins the lab!
Excited that Eden has decided to join the Gray Lab! Eden graduated from UC Irvine where she worked in the lab of Diane O’Dowd studying how mutations in the gene for the voltage-gated sodium channel NaV1.1 lead to epilepsy.
Let’s do some fun science together!
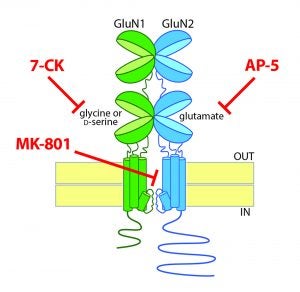
New review article on non-ionotropic NMDA receptor signaling
In collaboration with Karen Zito and Johannes Hell, I recently reviewed the emerging literature on possible non-ionotropic signaling by the NMDA receptor, which is classically a ligand-gated ion channel. This conformational-based signaling remains controversial but, if validated, could open the doors for a new era of understanding ion channels and synapse function.
Eden starts her rotation
My first paper at UC Davis
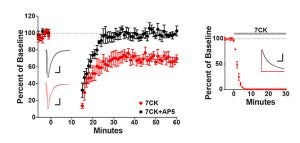
Was excited to be able to contribute to an elegant study from the Zito Lab showing that NMDA receptor-mediated spine shrinkage requires glutamate binding but not ion flux through the channel, supporting recent studies showing that the NMDA receptor can signal in an non-ionotropic manner in response to agonist binding.
John Gray awarded NARSAD Young Investigator Grant
 Our proposal entitled “Genetic ‘Saturation’ of the NMDA Receptor Glycine Co-Agonist Site” was selected for a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation. We are interested in understanding the fundamental basis for the unique requirement of NMDA receptors to bind both glutamate and a co-agonist (either glycine or D-serine). Even though our understanding is quite limited, in schizophrenia and other neuropsychiatric disorders, significant efforts have been made to clinically enhance NMDA receptor activity though this co-agonist site, either directly by high-dose administration of glycine, D-serine or D-cycloserine (a partial agonist) or indirectly though inhibition of glycine transporters or D-amino acid oxidase. We’re hoping that a deeper understanding of the mechanisms of NMDA receptor function and synapse biology will allow us to develop improved treatments for schizophrenia.
Our proposal entitled “Genetic ‘Saturation’ of the NMDA Receptor Glycine Co-Agonist Site” was selected for a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation. We are interested in understanding the fundamental basis for the unique requirement of NMDA receptors to bind both glutamate and a co-agonist (either glycine or D-serine). Even though our understanding is quite limited, in schizophrenia and other neuropsychiatric disorders, significant efforts have been made to clinically enhance NMDA receptor activity though this co-agonist site, either directly by high-dose administration of glycine, D-serine or D-cycloserine (a partial agonist) or indirectly though inhibition of glycine transporters or D-amino acid oxidase. We’re hoping that a deeper understanding of the mechanisms of NMDA receptor function and synapse biology will allow us to develop improved treatments for schizophrenia.
The Brain and Behavior Research Foundation is a leading non-profit dedicating to mental illness, please consider donating to this worthwhile charity.
New Pilot Grant from the ADC!
 I just received the exciting news that I have received a pilot project grant from the UC Davis Alzheimer’s Disease Center.
I just received the exciting news that I have received a pilot project grant from the UC Davis Alzheimer’s Disease Center.
Hippocampal synapse loss is an early finding in Alzheimer’s disease that is associated with elevated levels of soluble amyloid-β (Aβ) oligomers. These Aβ oligomers induce synaptic depression in the hippocampus in an NMDA receptor-dependent manner, but the mechanism remains unclear. My pilot project entitled “NMDA Receptor Subunit Dependence of Amyloid Beta-Induced Synaptic Depression” will use genetic approaches to examine the role of NMDA receptor GluN2 subunits in this Aβ-induced synaptic depression. The long-term goal is to understand the fundamental pathophysiology of Alzheimer’s disease at the earliest stages to allow for hypothesis-driven development of novel trajectory-altering treatments.